The Intricacies of mRNA Capping
This article delves into the crucial process of mRNA capping, a pivotal step in gene expression. mRNA capping is essential for mRNA stability, translation, and export from the nucleus. This process is significant in understanding cellular mechanisms and has implications for therapeutic developments, including mRNA vaccine technologies.
Introduction to mRNA Capping
The process of mRNA capping is crucial in eukaryotic gene expression. mRNA capping refers to the modification at the 5' end of the nascent RNA transcript, which has a profound impact on RNA stability, translation efficiency, and nuclear export. This biochemical event is vital to understanding cellular functions and is particularly relevant in the design and functionality of mRNA-based therapeutics, such as vaccines. The importance of mRNA capping cannot be overstated, as it plays a role in various cellular processes from transcription to translational regulation.
The Mechanism of mRNA Capping
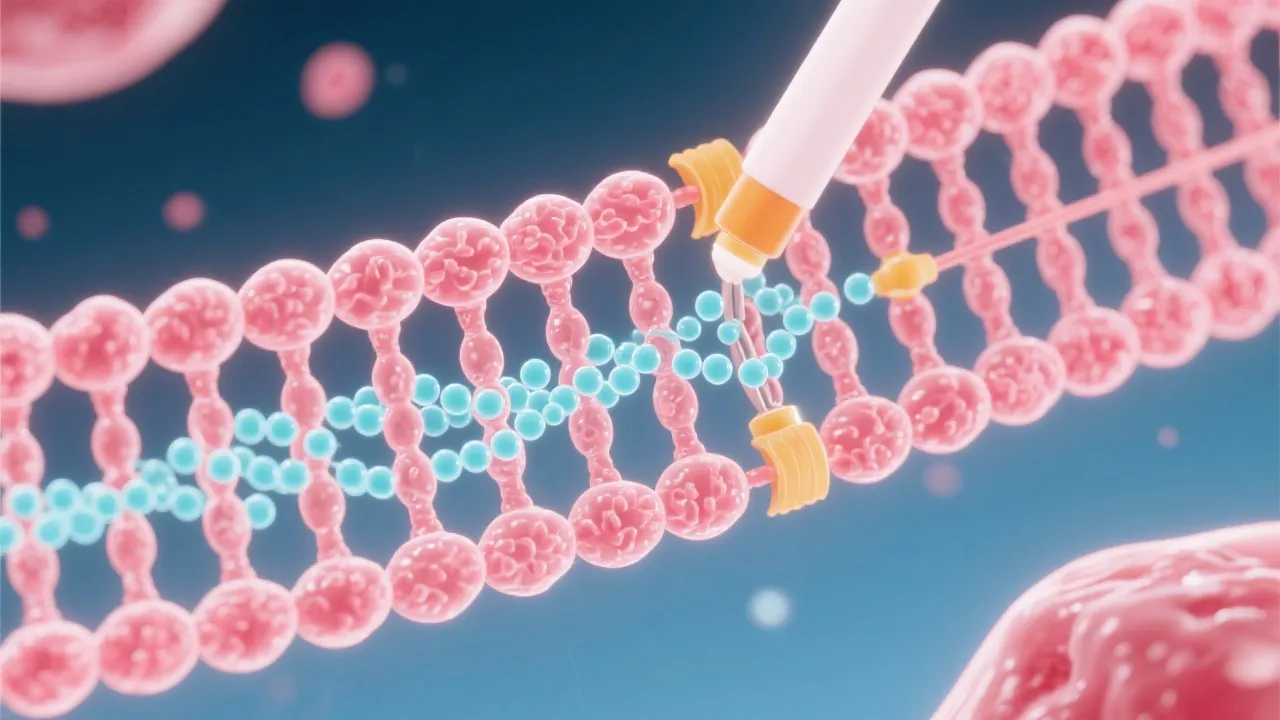
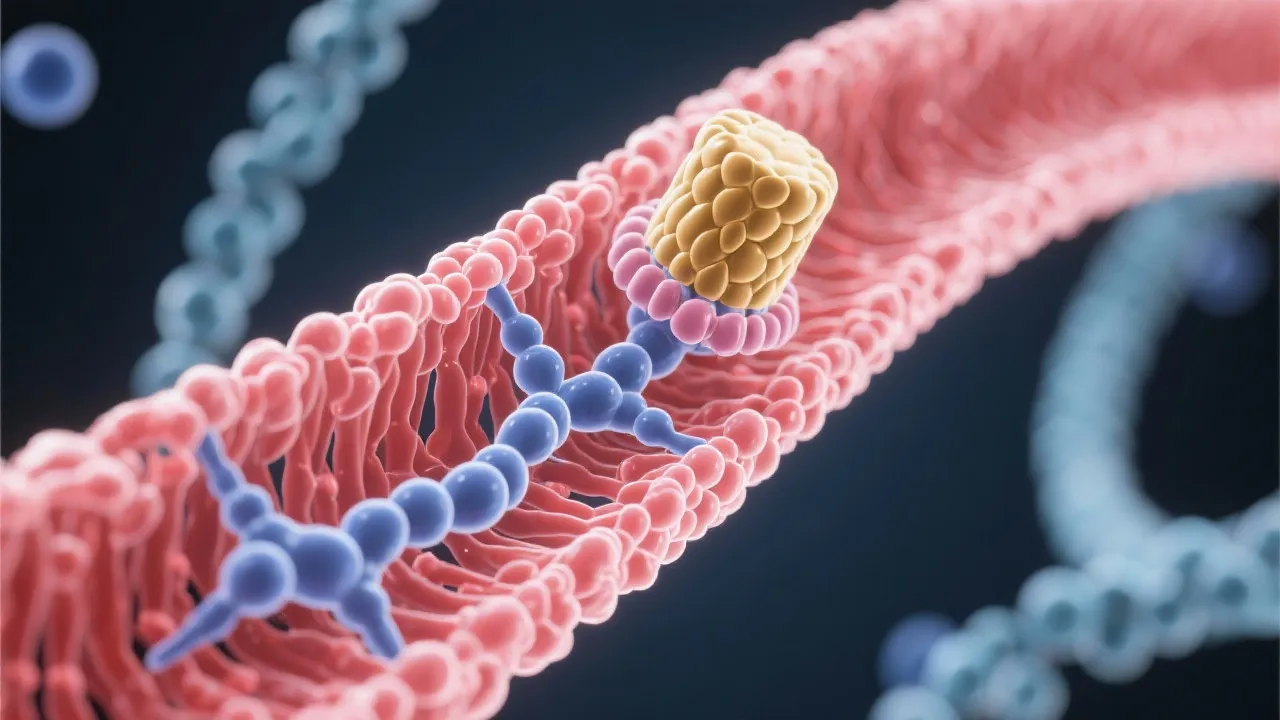
mRNA capping typically occurs in three sequential steps: the addition of a guanine nucleotide, followed by methylation of the guanosine cap, and further methylation at the ribose ring of the nucleotide. These modifications create a protective exonuclease-resistant structure that is crucial for the downstream processing of RNA transcripts. The initial step involves the enzyme guanylyltransferase, which facilitates the addition of a guanosine triphosphate (GTP) to the 5' end of the RNA transcript. This reaction leads to the formation of the unusual 5' to 5' triphosphate linkage that distinguishes capped mRNA from unmodified RNA. Following this, the cap structure undergoes methylation, primarily facilitated by methyltransferase enzymes, which convert the guanine into 7-methylguanosine, a key characteristic of the cap structure. The final step entails additional modifications to the ribose moiety, resulting in a fully mature and stable 5' cap.
Importance of mRNA Capping in Cellular Biogenesis
From an expert perspective, mRNA capping influences several cellular processes. Its impact is evident in mRNA's protection from exonucleases—enzymes that degrade RNA molecules—and its role in enhancing mRNA translation by aiding the ribosome’s recognition. This step is integral to the cell's protein synthesis machinery working efficiently. Protective mechanisms include creating an optimal structure for the ribosome binding and ensuring that RNAs are not recognized as foreign molecules by cellular surveillance systems. Furthermore, the cap structure guides RNA to the nuclear export machinery, thereby influencing the kinetics of mRNA in cytoplasmic activities, which is critical to proper gene expression and cellular homeostasis. By facilitating the timely and efficient export of mRNA molecules from the nucleus to the cytoplasm, capping enables the translation machinery to produce proteins essential for cellular functions.
mRNA Capping in Therapeutic Developments
The relevance of mRNA capping transcends basic cellular biology, marking its significance in medical sciences. Notably, mRNA vaccine technology, which has gained significant attention recently, depends on efficient capping for optimal performance. mRNA vaccines use synthetic mRNA designed to mimic naturally occurring capped mRNA, ensuring the body's cellular mechanisms recognize it effectively, prompting a robust immune response. Without proper capping, the synthetic mRNA would be subject to rapid degradation, thus reducing its effectiveness in eliciting the desired immune response. This has been observed in the development of various mRNA vaccines, particularly those targeting infectious diseases such as COVID-19. Moreover, mRNA capping has implications beyond vaccines; it also plays a role in cancer therapies, where modified mRNA can be employed to generate specific proteins that trigger immune checkpoints or enhance targeted drug delivery.
The Role of Enzymes in mRNA Capping
Central to understanding mRNA capping is acknowledging the enzymes involved. Guanylyltransferase begins the process by adding a guanosine triphosphate (GTP) to the nascent RNA, forming an atypical 5' to 5' triphosphate linkage. This unique structure is essential for protecting the RNA molecule from degradation. Subsequently, methyltransferase, utilizing S-adenosyl methionine (SAM) as a substrate, transfers a methyl group to the guanine. This enzymatic transformation of guanine into 7-methylguanosine characterizes the cap's defining structure, which fortifies the stability of mRNA. The precision and efficiency exhibited by these enzymes underscore the complexity of mRNA processing. Additionally, other associated enzymes and proteins play multifaceted roles in mRNA processing, transport, and turnover, including those involved in the splicing and polyadenylation of RNA; these processes are interconnected and critical for mature mRNA production.
Comparative Analysis: Standard mRNA vs. Synthetic mRNA Capping
To further our understanding, let's explore a comparison between naturally occurring mRNA capping and synthetic variants used in laboratory settings. This evaluation highlights specific distinctions in biochemical properties, application utility, and structural stability. Understanding these differences is critical, particularly in the context of therapeutic development and design.
| Aspect | Standard mRNA Capping | Synthetic mRNA Capping |
|---|---|---|
| Biochemical Process | Occurs naturally during transcription | Manually added during or after mRNA synthesis |
| Application | Cellular gene expression | Therapeutics and research |
| Stability | Extremely resistant to exonuclease decay | Designed for enhanced stability and translation |
| Regulatory Elements | Endogenous regulatory elements are present | May contain synthetic elements for enhanced translation |
| Delivery Mechanism | Internalized by natural cellular mechanisms | Requires delivery systems for cellular uptake |
Implications for Future Research
As scientific research advances, the study of mRNA capping remains a fertile ground for exploration. Understanding and manipulating this process offers promising avenues for innovations in gene therapy and personalized medicine. Further exploration into enzyme mechanisms, cap analogs, and their interaction with cellular machinery could usher in new scientific breakthroughs. The quest for optimizing mRNA for therapeutic applications includes the design of modified caps that improve the duration of mRNA stability and promote more efficient translation. Advances in nanotechnology, which could facilitate sophisticated delivery mechanisms for synthetic mRNA, also present exciting prospects. The nuances of the mRNA life cycle—including transcription, capping, splicing, export, translation, and degradation—provide a theoretically boundless framework for innovative therapies targeting a wide variety of diseases, from genetic disorders to complex conditions like cancer.
Clinical Applications of mRNA Capping
The application of mRNA capping in clinical settings has evolved dramatically in recent years. Beyond vaccines, therapies for genetically inherited diseases are being developed using mRNA technology where the correct protein can be delivered to the patient’s cells, potentially alleviating the symptoms of the disease. These therapies often rely on the same principles as mRNA vaccines, where robust immune responses can be trained by correctly processed mRNA. Furthermore, researchers are investigating the use of mRNA to produce therapeutic proteins, such as monoclonal antibodies or enzymes, for treating diseases. The robustness of mRNA systems has shown promise in producing proteins at unprecedented scales and de-risking the process of development by employing systems that can rapidly adapt to different proteins.
Challenges in mRNA Capping for Therapeutics
Despite its considerable advantages, there are challenges pertaining to mRNA capping that researchers must overcome. One of the key challenges lies in ensuring efficient delivery of synthetic mRNA to target cells, particularly in vivo. Traditional methods of delivering mRNA involve lipid nanoparticles, which can present issues with cellular uptake and endosomal escape. Additionally, ensuring the stability of synthetic mRNA during storage and transport remains a significant hurdle, as mRNA is inherently unstable and can degrade quickly. Moreover, the regulatory pathway for the approval of therapeutics based on mRNA technology is still evolving, requiring robust studies to assure safety and efficacy before they can be introduced commercially. Addressing these challenges will be critical in leveraging mRNA’s full potential in therapeutic settings.
FAQs
- What is mRNA capping?
mRNA capping is the modification of the 5' end of RNA transcripts, involving the addition of a guanine nucleotide and subsequent methylation, crucial for RNA stability and function. - Why is mRNA capping important?
It protects mRNA from degradation, enhances translation, and facilitates transport out of the nucleus, playing a vital role in gene expression. - How does mRNA capping relate to vaccines?
Efficient mRNA capping in synthetic mRNA is crucial for the stability and efficacy of mRNA vaccines, which are designed to elicit an immune response. - Are there other therapeutic uses for mRNA?
Yes, mRNA technology is being investigated for gene therapy, protein replacement therapies, and as a platform for producing therapeutic proteins and antibodies. - What challenges face mRNA therapeutic development?
Challenges include ensuring stable delivery of mRNA to target cells, improving storage stability, and navigating regulatory processes.
The Future of mRNA Technology
Looking ahead, the expansion of mRNA technology is set to revolutionize the fields of medicine and biotechnology. With ongoing research aimed at optimizing capping and addressing current challenges, we can anticipate a new era of precision medicine. Future mRNA applications may extend beyond conventional vaccination and treatment of infectious diseases to include broader applications in cancer immunotherapy, chronic disease management, and personalized genetic medicine. Innovations in gene-editing technologies, such as CRISPR, when combined with mRNA-based approaches, hold the potential to address some of the most challenging diseases affecting human health. As the field evolves, inter-disciplinary collaborations will be crucial in accelerating the translation of these scientific advancements into clinical practice, thereby enhancing patient outcomes and expanding the therapeutic possibilities of mRNA technology.