Understanding mRNA Capping Processes
mRNA capping is a crucial step in the post-transcriptional modification of eukaryotic messenger RNA, playing a vital role in mRNA stability and translation efficiency. This guide delves into the mechanisms behind mRNA capping, exploring its biological significance and the structures involved. It also examines recent advances and challenges facing researchers in this field.
Decoding mRNA Capping
One of the fundamental processes in cellular biology is the transcription and subsequent processing of messenger RNA (mRNA). A critical step in this sequence is mRNA capping, a modification that enhances the stability and translational efficiency of RNA. Understanding this process is essential for elucidating how genes are expressed and regulated within cells.
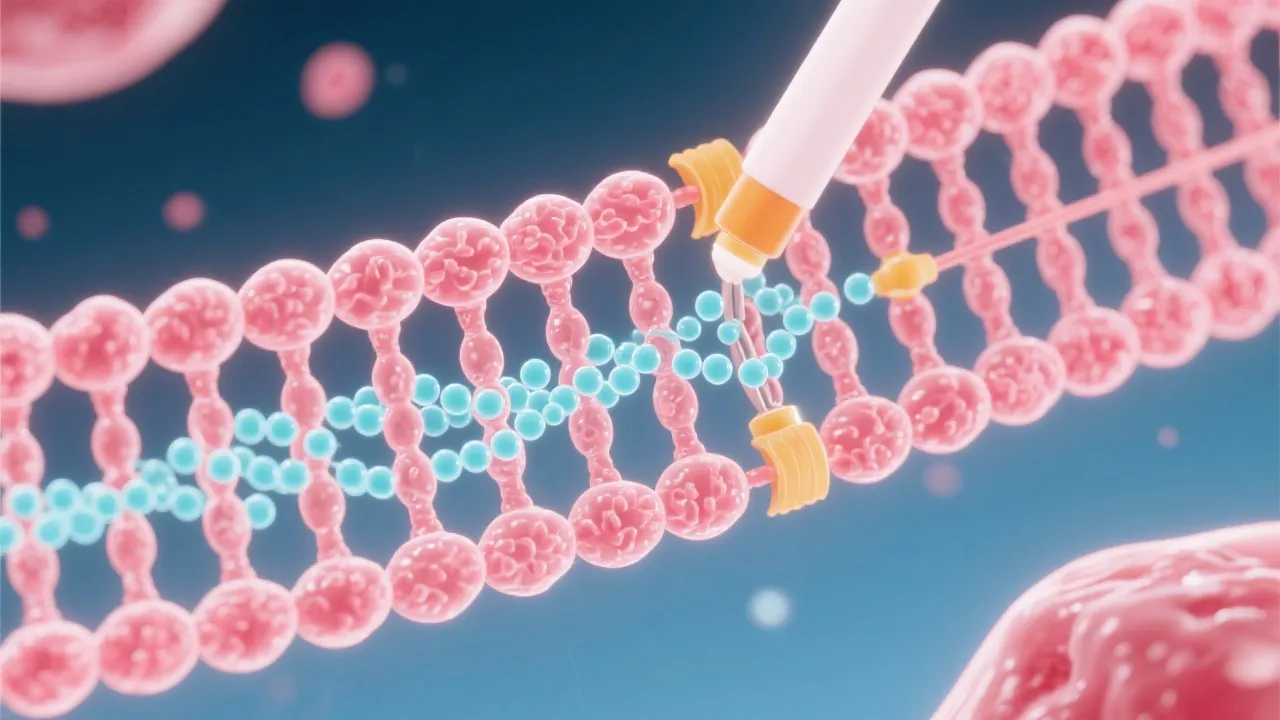
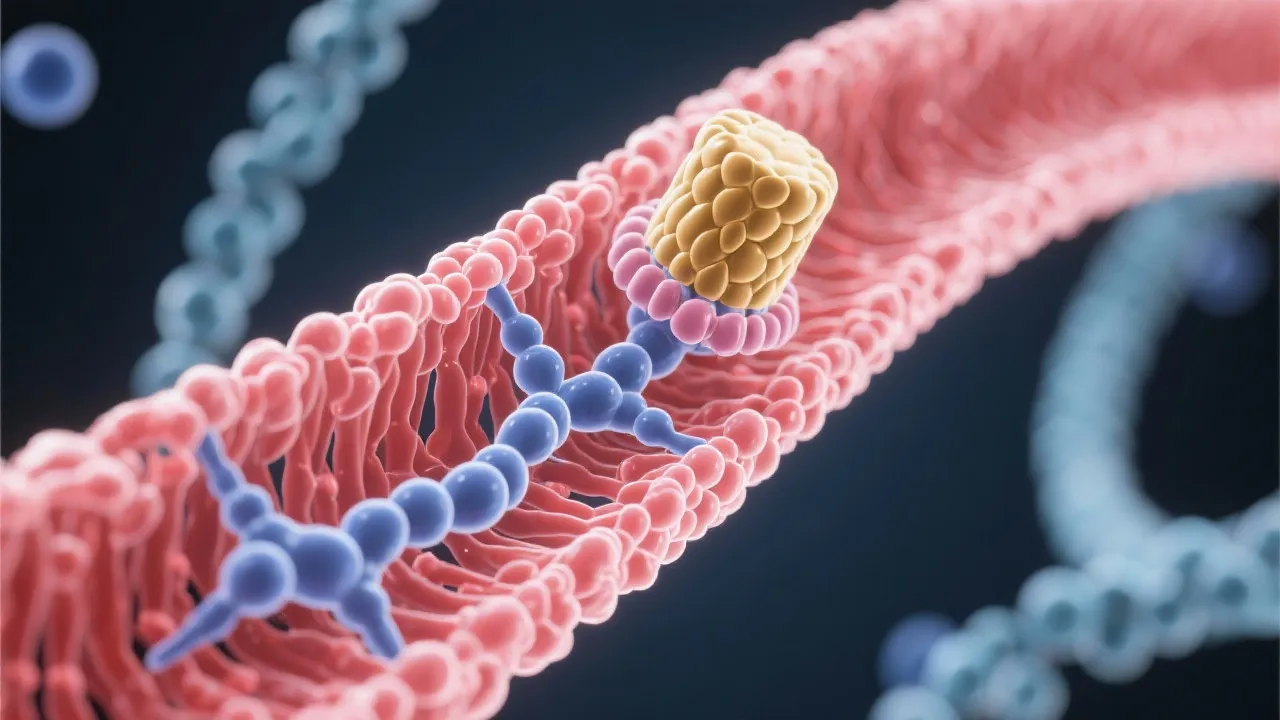
At its core, mRNA capping involves the addition of a 7-methylguanylate cap to the 5' end of the nascent RNA transcript. This cap structure is recognized by certain proteins that facilitate the export of the mRNA from the nucleus, aid in the initiation of translation in the cytoplasm, and protect the RNA from exonuclease-mediated degradation. Thus, mRNA capping is indispensable for proper RNA function and gene expression.
The Mechanisms Behind mRNA Capping
The process of capping occurs through three enzymatic reactions. It begins with the removal of the gamma phosphate from the 5' triphosphate end of the nascent RNA by RNA triphosphatase. Following this, a guanylyltransferase enzyme transfers a GMP moiety from GTP to the diphosphate end of the RNA, creating a 5'-5' triphosphate linkage. The final step involves a methyltransferase that adds a methyl group to the guanosine, forming the 7-methylguanylate cap.
Each of these steps is tightly regulated and coordinated by the capping enzyme complex, which associates with the RNA polymerase II phosphorylation site. This ensures that capping occurs efficiently and accurately as RNA polymerase transcribes the DNA into RNA.
What’s particularly interesting about this process is how it is intricately linked to the transcriptional machinery. As RNA polymerase II continues its work, the capping enzyme complex can quickly adjust to any changes, ensuring that mRNA is capped in real-time as it is being synthesized. This synchronous relationship between transcription and capping is critical for maintaining the integrity of the mRNA during its maturation process.
Biological Significance of mRNA Capping
The addition of the cap structure plays multiple roles in cellular biology. Very prominently, it influences mRNA stability. Without the cap, mRNA is highly susceptible to degradation by exonucleases, leading to the loss of gene expression. The cap also fosters ribosome binding during translation initiation, thereby enhancing the overall efficiency of protein synthesis.
Beyond structural benefits, mRNA capping also participates in the quality control mechanisms ensuring only correctly processed mRNAs are exported to the cytoplasm. Moreover, it interacts with the nuclear cap-binding complex (CBC) which aids in the transition of mRNA from transcription to processing and export phases.
The cap structure essentially acts like a signaling molecule. It indicates to the cellular machinery that the RNA molecule is ready for further processing and eventual translation. In addition, the cap is not solely a passive structure; it actively engages with various proteins that are vital for the RNA lifecycle. For instance, the interaction with the CBC not only protects the mRNA but also helps in the recruitment of spliceosomal components necessary for RNA splicing, thus forming a complex regulatory network.
Recent Advances and Challenges
Recent advancements in molecular biology and sequencing technologies have allowed for a deeper understanding of the nuances involved in mRNA capping. Research continues to explore variations in the capping process across different organisms and conditions, aiming to comprehend its roles in development, disease, and adaptation.
One of the central challenges facing researchers is the complexity of mRNA modifications and their interdependencies. mRNA capping does not occur in isolation; it is one of several post-transcriptional modifications that collectively determine the fate of an RNA molecule. Identifying the interplay between these modifications is key to understanding cellular homeostasis and response to environmental stimuli.
Moreover, the study of mRNA capping has implications in understanding various diseases, particularly cancer and genetic disorders. Changes in the capping process or capping enzymes often lead to aberrant mRNA expression patterns, which can drive tumorigenesis or contribute to developmental disorders. Therefore, by probing deeper into the mechanics of mRNA capping, researchers could potentially uncover new avenues for therapeutic intervention.
For example, certain inhibitors that target capping enzymes are being investigated as potential treatments for viral infections. Many viruses capitalize on the host's RNA capping machinery to stabilize their own transcripts, and disrupting this process could render them more susceptible to the immune response. This highlights the duality of mRNA capping—it is not only a feature of normal cellular processes but also a target for viral exploitation.
FAQs About mRNA Capping
- What is the primary function of the mRNA cap? The primary function of the mRNA cap is to protect mRNA from degradation, to aid in RNA export from the nucleus, and to initiate translation by facilitating ribosome binding.
- Why is the presence of the 5' cap crucial during translation? The 5' cap is essential during translation because it binds the cap-binding protein complex that aids ribosome attachment, thus increasing translation efficiency.
- Can mRNA function properly without a cap? Generally, an uncapped mRNA is rapidly degraded in the cell because exonucleases target it, leading to reduced gene expression.
- How do capping enzyme mutations affect cellular processes? Mutations in capping enzymes can severely impact RNA stability and translation, potentially leading to developmental defects or diseases owing to disrupted gene expression.
- How does mRNA capping differ between eukaryotic and prokaryotic organisms? Eukaryotic organisms process mRNA with a capping mechanism, whereas prokaryotic organisms generally do not utilize such capping processes, relying instead on different RNA stabilization techniques.
- What ongoing research exists regarding therapeutic applications of mRNA capping? Ongoing research explores the possibility of targeting capping enzymes for drug development, especially in treating diseases characterized by aberrant mRNA processing, such as cancers and genetic disorders.
Conclusion
mRNA capping is a pivotal process in the post-transcriptional regulation of gene expression. Through evolutionary conserved mechanisms, this modification ensures that mRNA is not only stable and translatable but also appropriately processed and exported to the cytoplasm. As research advances, understanding the intricacies of mRNA capping will provide further insights into its role in cellular and organismal biology, and potentially unravel new therapeutic targets for genetic diseases.
Furthermore, the continued exploration of mRNA capping can enhance our understanding of synthetic biology applications, where researchers strive to engineer mRNA for therapeutic purposes, streamline vaccine development, and explore novel treatments for various ailments. As scientists unravel the complexities of this essential biological process, it ultimately contributes to our broader comprehension of life at the molecular level, further bridging gaps between genetics, molecular biology, and therapeutic applications in medicine.
Future Directions in mRNA Capping Research
Looking forward, the landscape of research on mRNA capping is set to expand significantly. As technologies such as CRISPR and single-cell sequencing become more sophisticated, they pave the way for unprecedented insights into how capping influences gene regulation across different tissues and developmental stages.
Moreover, the role of mRNA capping in circadian rhythms and stress responses is an emerging area of study. Investigating how capping modifications adapt to various physiological conditions could shed light on the dynamics of gene expression during stress, inflammation, and environmental changes.
In the realm of virology, understanding the specifics of how viruses hijack host mRNA capping mechanisms opens doors for innovative antiviral strategies. Researchers are keen to uncover the differential strategies used by various viruses, leading to the development of broad-spectrum antiviral agents targeting these capping processes.
In conclusion, as mRNA capping continues to be a focal point of scientific inquiry, it holds the promise of reshaping our understanding of gene regulation, disease mechanisms, and therapeutic innovations. The journey of mRNA capping research not only enriches our knowledge of molecular biology but also inspires new tools and strategies for addressing complex biological challenges in human health.