Understanding mRNA Capping Process
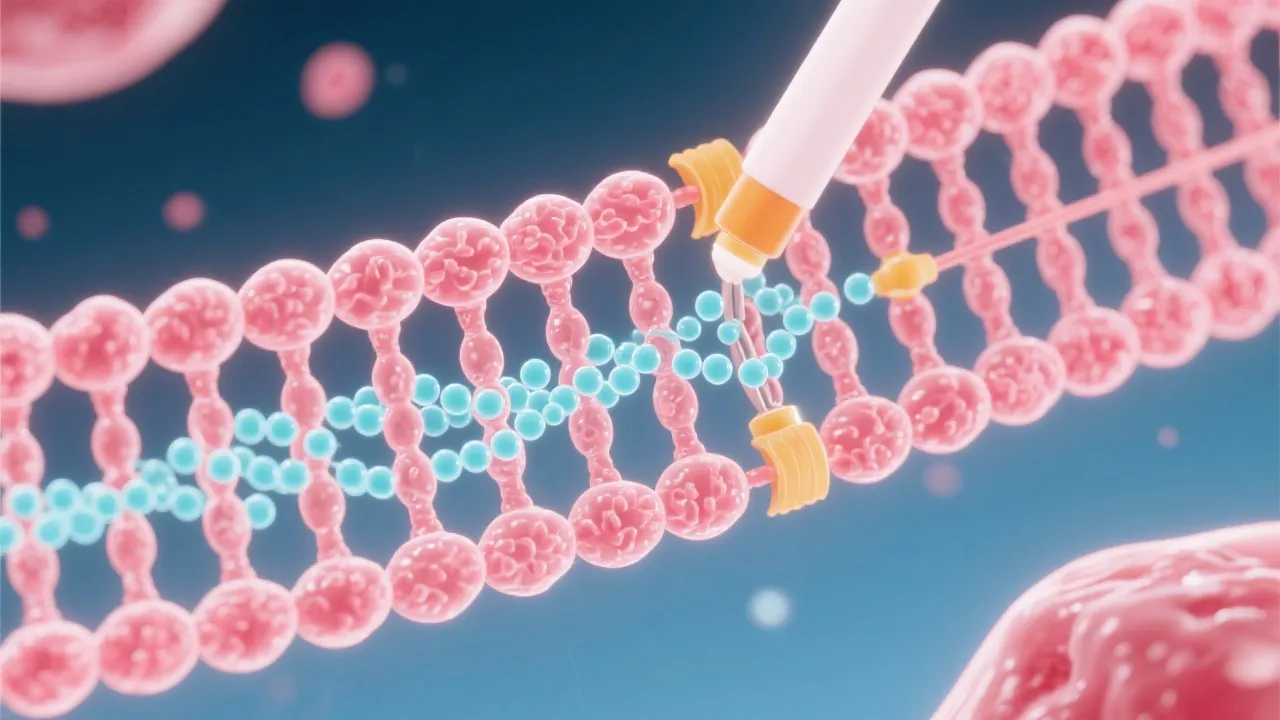
mRNA capping is a crucial molecular process that modifies the 5' end of the nascent mRNA transcript. This cap structure is vital for mRNA stability, nuclear export, and efficient translation in eukaryotic cells. Understanding the intricacies of mRNA capping can shed light on its role in gene expression and its potential impact on therapeutic interventions.
An In-Depth Look at mRNA Capping
The process of mRNA capping is an essential modification that occurs in eukaryotic cells, specifically at the 5' end of the nascent pre-mRNAs. This modification involves the addition of a modified guanine nucleotide which is the stepping stone into a mature mRNA molecule that can efficiently participate in protein synthesis. The importance of mRNA capping cannot be overstated, as it plays a central role in mRNA stability, splicing, transport, and translation. Furthermore, the cap structure is fundamental for translational accuracy, as it directly influences ribosomal binding and initiation of the protein synthesis machinery.
The Biochemical Pathway of mRNA Capping
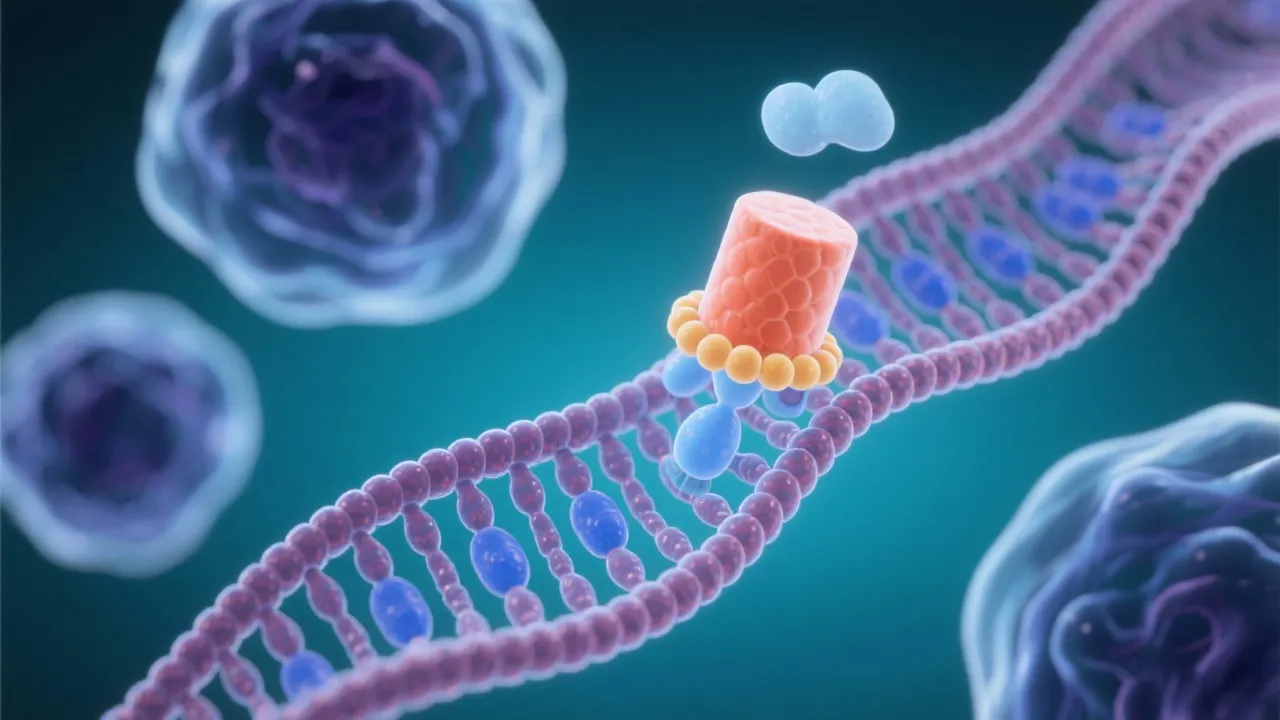
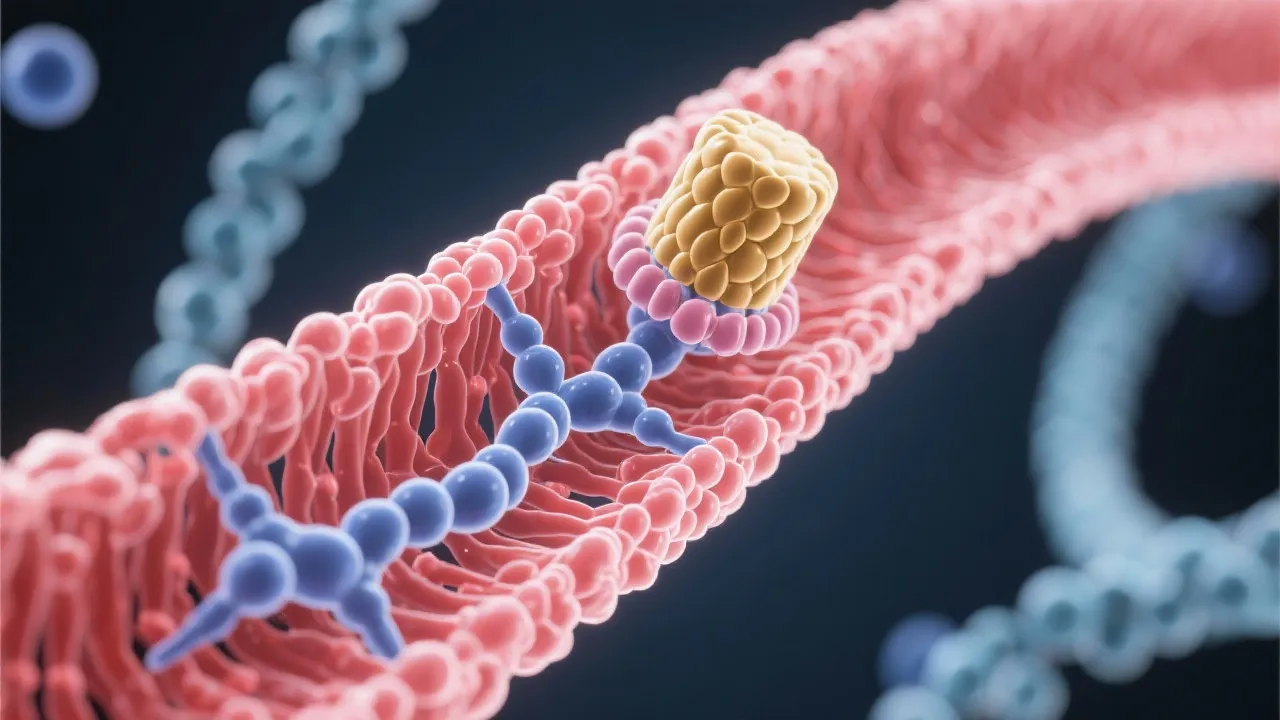
The mRNA capping process is initiated shortly after the commencement of transcription. The enzyme guanylyltransferase catalyzes the attachment of a GMP - derived from GTP - to the precursor mRNA, in a 5’-5’ triphosphate linkage. This enzymatically catalyzed process ensures that the cap structure, typically referred to as the 7-methylguanylate cap, is properly affixed. The 5'-5' triphosphate linkage is rather unique and provides a stable attachment site that is not easily degraded by exonucleases. Further modifications by methyltransferase enzymes add methyl groups, typically at the N7 position of the guanosine and the 2'-O position of the first nucleotide of the mRNA. This additional methylation not only protects the mRNA but also enhances its interaction with various cellular factors that are critical for mRNA functionality.
The cap structure is uniquely recognized by cap-binding proteins such as CBP20 and CBP80, which form part of the cap-binding complex (CBC). This complex plays an important role in various downstream processes, such as mRNA export from the nucleus, splicing, and initiation of translation. These proteins are vital in helping the mRNA evade degradation by the exonucleases that are constantly active in the cellular environment, thereby ensuring the stability of the mRNA transcript long enough for successful translation into protein.
The Role of mRNA Capping in Gene Expression
Understanding the role of mRNA capping in gene expression illuminates its broader implications in cellular function and health. An efficient capping process augments mRNA stability, protecting it from exonucleolytic degradation. Without this protection, mRNA transcripts would quickly degrade, reducing the amount of protein produced from each transcript. The cap is also pivotal for the proper assembly of the ribosome on the mRNA; the cap-binding proteins facilitate the recruitment of the ribosomal subunits, guaranteeing an accurate and efficient translation process. This interaction is crucial because any lapses can lead to mistranslation or no translation at all, which can severely impact cellular function.
Moreover, the capping process is closely associated with other post-transcriptional modifications, such as splicing and polyadenylation, highlighting the intricate coordination required for effective gene expression. Mutations or malfunctions in the capping process can severely disrupt these cellular events, potentially resulting in diseases or developmental disorders. For instance, certain mutations in the guanylyltransferase enzyme can lead to defects in the capping process, resulting in a range of disorders characterized by ineffective protein synthesis.
The Therapeutic Potential of Targeting mRNA Capping
Given its centrality in gene expression, mRNA capping has emerged as a candidate for therapeutic targeting, particularly in viral infections and cancer. Inhibitors of the capping process can be utilized to prevent viral replication, as many viruses, including Influenza and Ebola, rely heavily on the host’s capping mechanisms for their mRNA to be translated into viral proteins. The viruses typically encode proteins that can manipulate or hijack the host’s capping enzymes, meaning that targeting capping could effectively reduce viral load. Likewise, targeting the capping machinery can be a strategy to selectively influence cancerous cells, which may exhibit dysregulation in capping activities.
The therapeutic implications extend to the use of small molecules that can disrupt the capping process, as well as larger therapeutic agents designed to alter capping pathways selectively. For example, research has indicated that certain compounds that inhibit the activity of methyltransferases can affect mRNA stability and protein expression in cancer cells, making them potential treatments. Such innovative strategies could also extend the promise of mRNA vaccines, where enhanced capping improves vaccine effectiveness by increasing the stability and translating efficiency of the mRNA produced in the vaccine formulation.
Activity and Regulation of Capping Machinery
The activity of the capping machinery is tightly regulated to ensure a proper response to cellular conditions. This regulatory aspect is crucial, as the capping process is initiated almost immediately during transcription. Factors such as RNA polymerase II, which is responsible for synthesizing mRNA, also play a significant role in cap addition. The capping enzyme is recruited to the polymerase during transcription, which highlights the integrated nature of the transcription and capping processes.
Regulation of the capping process can occur through various mechanisms, including phosphorylation events and the cellular availability of nucleotide substrates. For instance, transcription factors can enhance or inhibit the recruitment of capping enzymes depending on the cellular context and external signals such as growth factors and stress responses. Various cellular stresses can change the dynamics of RNA processing and capping, providing a point of control in gene expression. These regulatory networks illustrate the complexity of mRNA biology and the fine balance eukaryotic cells maintain to respond to environmental cues while ensuring proper gene expression.
mRNA Capping and Its Implications in Disease
The implications of mRNA capping extend well into the realm of disease research and understanding. Aberrations in mRNA capping machinery can lead to a myriad of health issues, including various types of cancer, neurodegenerative diseases, and even genetic disorders. Research has shown that certain cancers are associated with mutations in genes encoding enzymes involved in the capping process. These mutations can result in the production of improperly capped mRNAs, leading to the translation of malfunctioning proteins that promote cancer cell survival and proliferation.
In neurodegenerative diseases, altered mRNA capping has been implicated in the loss of neuronal function and survival. For instance, some studies suggest that improper capping of mRNAs essential for neuronal health can lead to reduced expression of neuroprotective proteins, making neurons more susceptible to apoptosis or degeneration. The relationship between mRNA capping and disease highlights the need for further exploration into this essential biological process, as it presents potential avenues for therapeutic intervention.
Current Research and Future Directions in mRNA Capping
Research into mRNA capping has expanded significantly in recent years, particularly with technological advancements such as high-throughput sequencing and proteomics that allow for a detailed understanding of capping dynamics in various cellular contexts. Additionally, studies are increasingly focusing on how this modification interacts with other RNA modifications and how these cumulative effects influence gene expression.
Future research directions may involve investigating alternative capping pathways that exist in certain cellular conditions or organisms. Understanding how different organisms may utilize variations of the capping mechanism could offer insights into evolutionary biology and the adaptation of cellular processes. Furthermore, the relationship of mRNA capping with emerging RNA-based technologies, including CRISPR and RNA interference, represents a promising area for future exploration, possibly leading to new techniques to manipulate gene expression or treat diseases.
Emerging Technologies and Their Impact
The advent of CRISPR technology, RNA-editing tools, and next-generation sequencing has opened up new horizons in the study and manipulation of mRNA capping. These technologies allow researchers to edit the genetic material more precisely, leading to advanced understandings of the role of capping in RNA metabolism. They also provide a novel framework to investigate how modulating the capping mechanism can influence gene expression directly.
In particular, CRISPR-based approaches can be utilized to explore the effects of specific mutations in genes encoding capping proteins. Researchers can create precise knockouts or modifications to evaluate the downstream consequences of capping dysregulation. This capability accelerates our capacity to dissect complex pathways that contribute to diseases tied to mRNA processing and may lead to innovative strategies that target capping for therapeutic purposes.
FAQs
- What is mRNA capping?
mRNA capping is a biochemical modification that occurs at the 5' end of a nascent mRNA transcript, involving the addition of a modified guanine nucleotide and methyl groups. - Why is mRNA capping important?
mRNA capping is crucial for mRNA stability, nuclear export, translation efficiency, and protection from exonucleolytic degradation. The cap structure also facilitates ribosomal binding to initiate translation. - How does mRNA capping affect translation?
The cap structure allows cap-binding proteins to facilitate the proper assembly of the ribosome, ensuring accurate translation. It serves as a signal for the ribosomal proteins that participate in translation initiation. - Can mRNA capping be targeted therapeutically?
Yes, inhibiting capping processes is being explored as a strategy to manage viral infections and cancer. Modulating the capping apparatus provides a promising method to selectively affect diseased cells. - What diseases are associated with impaired mRNA capping?
Aberrations in mRNA capping have been linked to various cancers, neurodegenerative diseases, and genetic disorders due to the disturbance of normal protein synthesis and stability. - What role do emerging technologies play in mRNA capping research?
The development of technologies such as CRISPR and RNA sequencing facilitates detailed studies on mRNA capping dynamics and enables the potential manipulation of this process for therapeutic development.
Conclusion
The scientific understanding of mRNA capping extends beyond basic biology, demonstrating its significance in health and disease. As research continues to deepen our knowledge of this intricate process, the potential for innovative applications in medicine and biotechnology becomes increasingly apparent. The overlap between mRNA capping research and technology offers exciting prospects for future advancements, promising a frontier for exploration that could ultimately transform therapeutic strategies across various medical fields.